
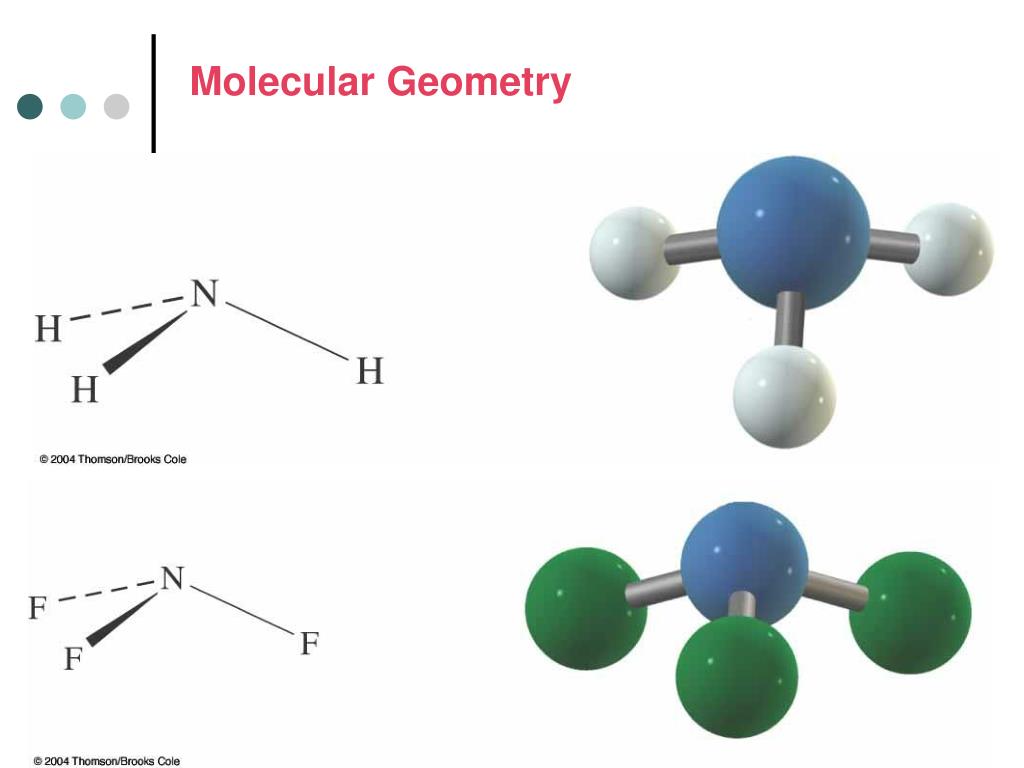
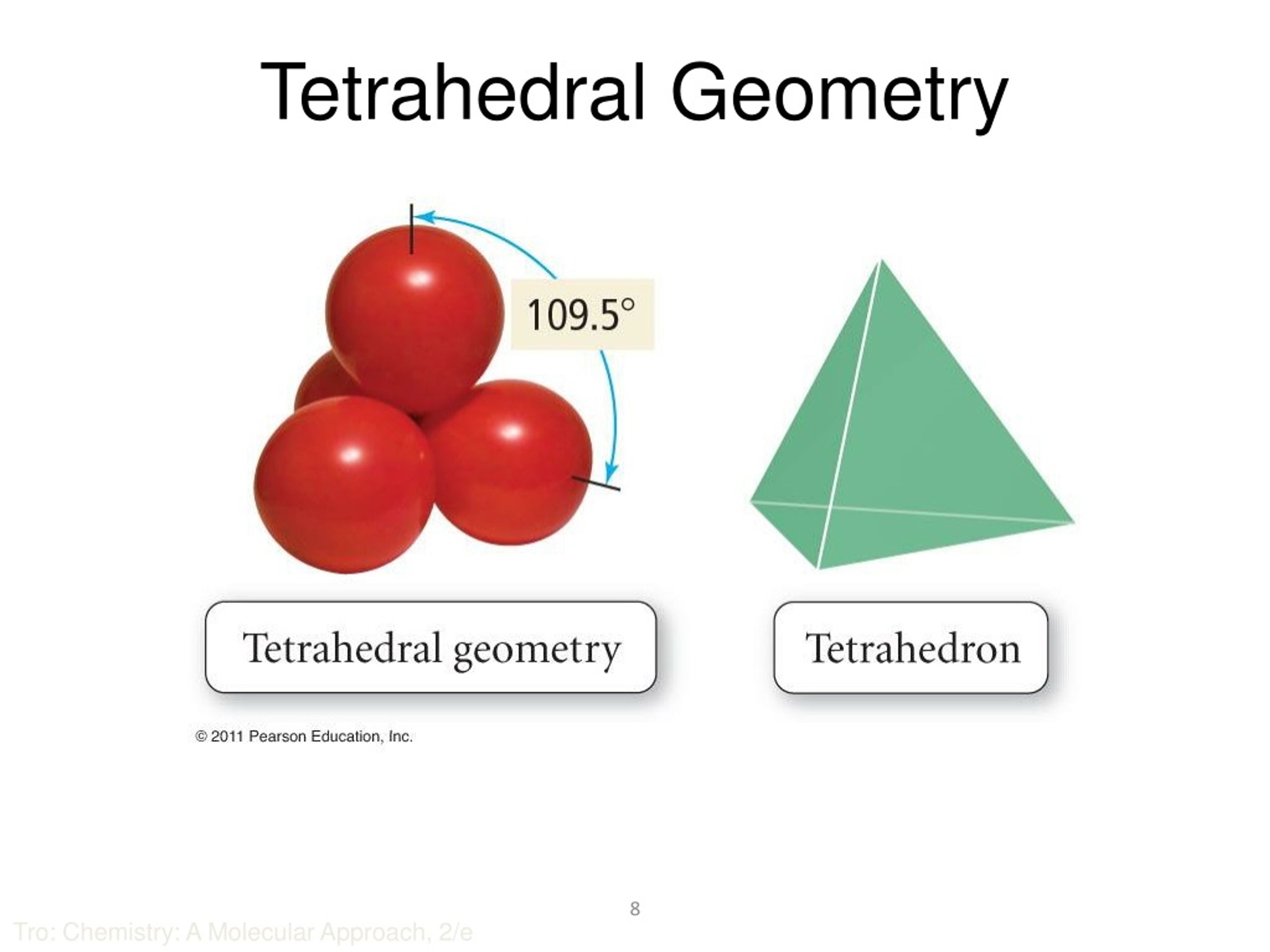
It is a group 14 hydride and the simplest alkane, and is the main component of natural gas. In contrast, boron trifluoride is flat, adopting a trigonal planar geometry because the boron does not have a lone pair of electrons. Methane (US /men/ or UK /mien/) is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms of hydrogen). However, the three hydrogen atoms are repelled by the electron lone pair in a way that the geometry is distorted to a trigonal pyramid (regular 3-sided pyramid) with bond angles of 107°. This would result in the geometry of a regular tetrahedron with each bond angle equal to cos −1(− 1 / 3) ≈ 109.5°. The nitrogen in ammonia has 5 valence electrons and bonds with three hydrogen atoms to complete the octet. Phosphine, an example of a molecule with a trigonal pyramidal geometry. If these are all bond pairs the molecular geometry is tetrahedral (e.g. We'll use VSEPR Theory and the Lewis Structure for CH4 and. For example four electron pairs are distributed in a tetrahedral shape. The AXE method for VSEPR theory states that the classification is AX 3E 1. In this video we'll look at the Tetrahedral Molecular Geometry and Bond Angles. In organic chemistry, molecules which have a trigonal pyramidal geometry are sometimes described as sp 3 hybridized. Some molecules and ions with trigonal pyramidal geometry are the pnictogen hydrides (XH 3), xenon trioxide (XeO 3), the chlorate ion, ClO −ģ. When all three atoms at the corners are identical, the molecule belongs to point group C 3v. In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as shown in Figure 9.3 'Tetrahedral Geometry'. 1984 17(11) pp 379 - 386 doi:10.Molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base Trigonal pyramidal molecular geometry From an electron group geometry perspective, GeF 2 has a trigonal planar shape, but its real shape is dictated by the positions of the atoms. ^ Inverted geometries at carbon Kenneth B.These electron pairs are involved in the. The Carbon atom in methane is surrounded by four electron pairs. The other molecular geometries are collected according to the AXE method. Examples of Tetrahedral Structured Molecules.For carbon this phenomenon can be observed in a class of compounds called the fenestranes. Note that inversion also takes place in so-called Walden inversion and nitrogen inversion but with different meanings.Ī tetrahedron can also be distorted by increasing the angle between the two opposite bonds (again by force) resulting in the extreme case in complete flattening. The penalty usually is increase in strain energy for the molecule resulting in increased reactivity. Organic molecules displaying inverted carbon are tetrahedranes and propellanes. In inverted carbon for instance all 4 substituents are now on the same side.
To know the total valence electron in the NBr3 molecule, we get to know the valence electron of an individual atom (nitrogen and bromine). The octahedron is one of the Platonic solids. Count total valence electron in NBr3 Valence electrons are usually the electrons found in the outermost shell of an atom. The octahedron has eight faces, hence the prefix octa. There are several shapes in molecular geometry, but in this lesson, we'll focus on the tetrahedral.

Figure 7.23 (a) H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. In chemistry, octahedral molecular geometry, also called square bipyramidal, 1 describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. Molecular geometry is a type of geometry used to describe the shape of a molecule. Geometrical constraints in a molecule may cause a severe distortion of a tetrahedral geometry towards an inverted one. Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5.


 0 kommentar(er)
0 kommentar(er)
